Microscopy and Histology Suite
Our Microscopy and Histology Suite hosts a wide range of specimen preparation and advanced microscopy equipment to support biomedical research. Training and expertise in the use of instruments are provided by our staff.
The Dragonfly is our newest high-speed and high-sensitivity microscope for various sample types, from large 3D specimens to single cells. The Dragonfly offers high resolution, fast 3D imaging and seamless large-area stitching.
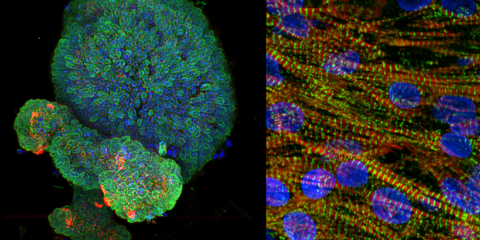
Features
- Large Area Stitching:
- Borealis Field Uniformity and Imaris Stitcher module
- 3D imaging
- Choice of pinhole sizes 25um or 40um, fast Piezo Z stage and fast camera triggering
- High Resolution
- Choice of 2 high-sensitivity cameras: Sona sCMOS (2048x2048px) and iXon 888 EMCCD (1024x1024px)
- Highest quality Nano Crystal Coat Nikon objectives
- Motorised camera magnification (1x, 1.5x, 2x) and GPU accelerated Deconvolution
- SRFF stream super-resolution
- Live Cell Incubation
- MicroPoint photo-ablation
- Brightfield and DIC
- Widefield Laser Fluorescence
Excitation and Emission
| wdt_ID | Laser Excitation (nm) | Dichroic | Emission Filters (nm) |
|---|---|---|---|
| 1 | 405 | 1 | 445/46 |
| 2 | 445 | 2 | 478/37 |
| 3 | 488 | 1 | 521/38 |
| 4 | 514 | 2 | 538/20 |
| 5 | 561 | 1 | 594/43 |
| 6 | 637 | 1 | 685/47 |
| 7 | 730 | 1 | 809/90 |
Objectives
| wdt_ID | x | Immersion media | Correction | Numerical Aperture (NA) | Working Distance (mm) | Coverglass thickness (mm) |
|---|---|---|---|---|---|---|
| 1 | 4x | Dry | CFI Plan Apo Lambda | 0,20 | 20,00 | |
| 2 | 10x | Dry | CFI Plan Apo Lambda | 0,45 | 4,00 | 0.17 |
| 3 | 20x | Dry | CFI Plan Apo VC | 0,75 | 1,00 | 0.17 |
| 4 | 25x | Silicone Oil | CFI Plan Apo Lambda | 1,05 | 0,55 | Correction collar |
| 5 | 0.11-0.23, Set at 0.17 | |||||
| 6 | 40x | Oil | Plan Fluor | 1,30 | 0,24 | 0.17 |
| 7 | 60x | Oil | Plan Apo Lambda | 1,40 | 0,13 | 0.17 |
Contacts and training
To discuss how the Dragonfly Confocal Microscope could support your research, please email michael.nesbit@curtin.edu.au.
The Zeiss Axioscan Z.1 Digital Slide Scanner is ideal for large-area batch imaging of large experiments. This automated system can scan 100 slides in transmitted brightfield, cross-polarised light and 7 channels of widefield fluorescence.
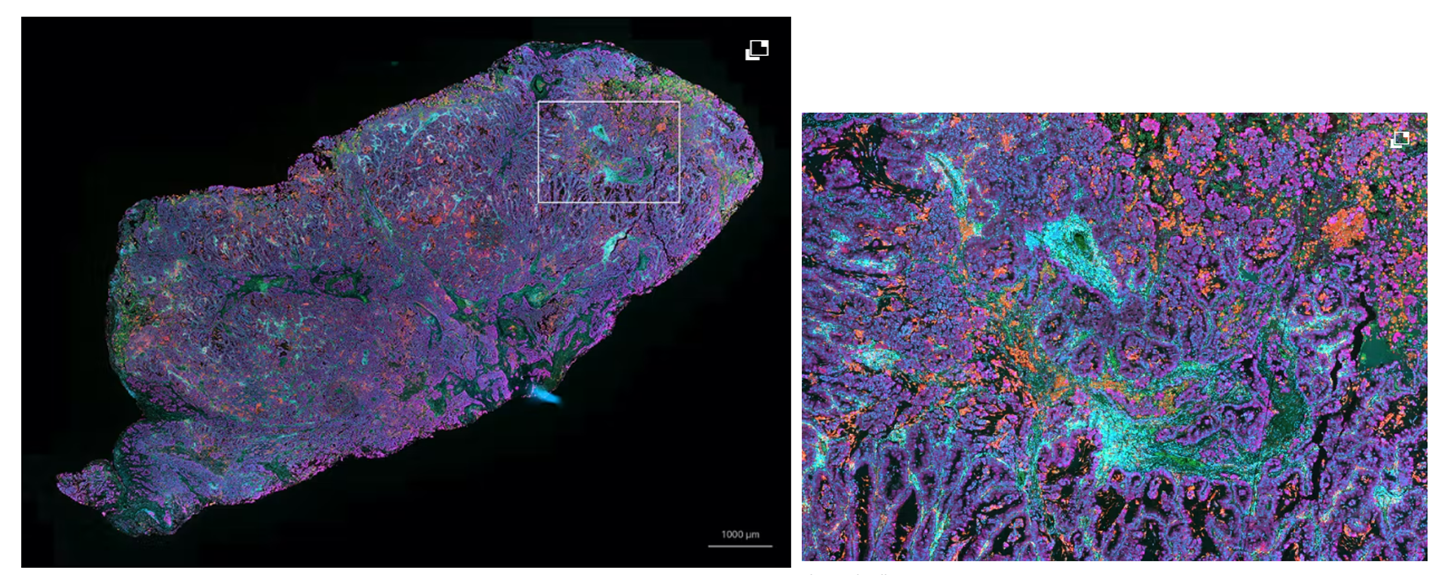
Features
-
- LED excitation allows for high-speed imaging with minimal mechanical filter switching
- Penta-band filter for DAPI, GFP, Cy3, Cy5, Cy7 and tri-band filter for CFP, YFP and mCherr
- 2x High-end cameras for brightfield in colour and high-sensitivity monochrome for fluorescence.
- Extra narrow band emission filters and spectral unmixing for 7 colour imaging (e.g. Opal)
- Automatic focus mapping to ensure that entire samples are captured in focus even at high magnification
- Z stack acquisition with extended depth of focus algorithms for thicker tissues or deconvolution for signal enhancement
- ZEN 3.1 Blue Software, including integrated image analysis
- 2x offline workstations including Intellesis machine learning segmentation module, colocalisation analysis and Third-Party Import
Excitation and Emission
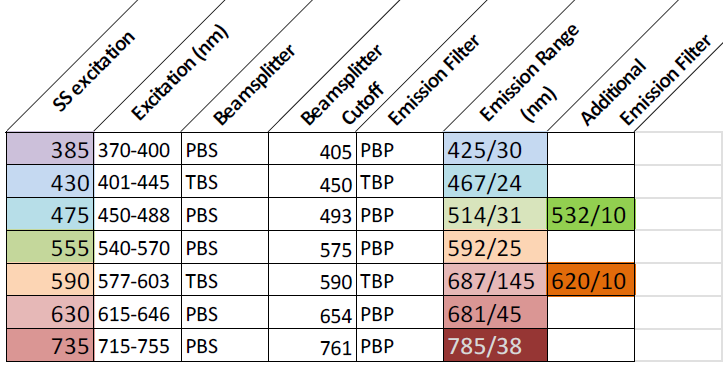
Objectives
5x, 10x, 20x, 20x POL and 40x corr (dry)
Contacts and training
To discuss how the Zeiss Axioscan Z.1 could support your research, please contact michael.nesbit@curtin.edu.au.
The Nikon A1+ Laser Scanning Confocal is our versatile and high-performance microscope, enabling various techniques, including multicolour imaging, fast live imaging and spectral detection. This microscope is user-friendly for basic imaging and highly configurable for advanced applications or complex samples.
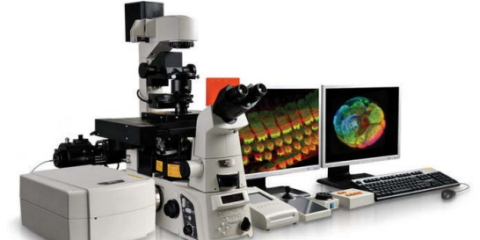
Features
- Four lasers (405nm, 488nm, 561nm, 640nm)
- Four filters (450/50, 525/50, 595/50, 700/75)
- Four PMT detectors
- Spectral detectors all operated using NIS-Elements software.
- CCD Camera for Brightfield, DIC and Widefield applications
- Motorised stage and High Content Analysis module for multi-well plates
- Range of objectives available (10x up to 100x; dry, water, oil and multi-immersion options)
- Capacity for live cell imaging with a Tokai stage top incubator and perfect focus system.
Excitation and Emission
| wdt_ID | Laser Excitation (nm) | Emission Filters (for PMT) |
|---|---|---|
| 1 | 405 | 450/50 |
| 2 | 488 | 525/50 |
| 3 | 561 | 595/50 |
| 4 | 640 | 700/755 |
Objectives
| wdt_ID | x | Immersion Media | Correction | Numerical Aperture (NA) |
|---|---|---|---|---|
| 1 | 10x | Dry | PlanApo | 0,45 |
| 2 | 20x | Dry | PlanApo | 0,75 |
| 3 | 20x | Multi-immersion | PlanFluor | 0,75 |
| 4 | 40x | Dry | PlanApo | 0,95 |
| 5 | 60x | Water | PlanApo | 1,20 |
| 6 | 100x | Oil | PlanApo | 1,45 |
Funding/ Disclaimer
NA
Contacts and training
To discuss how the Nikon A1+ Confocal could support your research, please contact michael.nesbit@curtin.edu.au.
The Ultraview Spinning Disk Confocal microscope is a high-speed, high-sensitivity system for quickly acquiring 2D and 3D images of tissues on slides or cells in dishes. The instrument’s fast speed makes it useful for multi-point image acquisition.
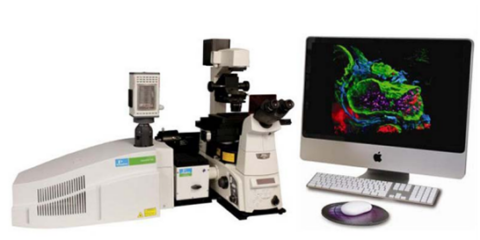
Features
- Motorised stage and focus
- 2 EMCCD cameras for signal amplification and dual-camera acquisition
- Good objectives for both low and high-magnification imaging
Excitation and Emission
| wdt_ID | Laser Excitation (nm) | Emission Filters (nm) |
|---|---|---|
| 1 | 405 | 445/60 and 615/70 |
| 2 | 440 | 485/60 |
| 3 | 488 | 525/50 |
| 4 | 514 | 595/50 |
| 5 | 561 | 614/70 |
| 6 | 640 | 700/75 |
Objectives
| wdt_ID | x | Immersion Media | Correction | Numerical Aperture (NA) |
|---|---|---|---|---|
| 1 | 4x | |||
| 2 | 10x | Dry | PlanFluor | 0,30 |
| 3 | 20x | Dry | UplxApo | 0,95 |
| 4 | 40x | Dry | Uplx Apo x40 | 0,80 |
| 5 | 60x | Water | PlanApo | 1,20 |
| 6 | 100x | Oil | PlanApo | 1,40 |
Contacts and training
To discuss how the Ultraview could support your research, please contact michael.nesbit@curtin.edu.au.
We have manual Widefield microscopes for routine and short session use which can be used without advance booking.
Olympus Widefield Microscopes
Transmitted light (brightfield) and standard fluorescence filters (visible spectrum) are available for both. The Olympus Upright IX-51 is ideal for slides and has excellent Brightfield Optics to set up Kohler Illumination. The Inverted IX-51 is ideal for slides and has longer working distance objectives with correction collars and phase contrast at 10x. Both microscopes have a colour CCD linked to CellSens acquisition software.

Zeiss Axioskop 2 with Nuance Camera
This manual upright system has standard fluorescence filter cubes and excellent brightfield illumination. The Nuance camera is a monochrome spectral camera that is ideal for separating multiple IHC stains in tissue sections by spectral unmixing and removal of autofluorescence using the inForm analysis software.
Instrument specifications | Olympus Microscopes Configuration Guide [.pdf 140KB]
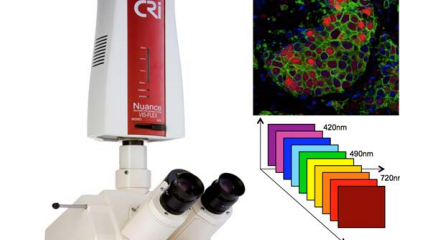
Image Visualisation and Analysis
Increasingly large data sets and sample numbers are being captured on our microscopes; however, critical information often needs to be extracted through image processing and analysis pipelines.
Our Researchers have access to several high-end software packages on dedicated computer workstations for image visualisation and data analysis.
Licensed Software for Image Visualisation and Analysis
ZEN (Zeiss)
Zeiss ZEN software is used to view, process and export large 2D image files from the Axioscan. We have licensed powerful modules, including:
- Image analysis
- Intellesis machine learning segmentation
- Advanced processing and analysis
- Co-localisation.
Third party import enables analysis of images captured on other microscopes. Large datasets can be quantified directly into Microsoft Excel documents using Batch Analysis.
These modules enable simple measurements of features in 2D datasets, including counting, intensity, double positives and morphology parameters. In addition, Intellesis is a machine learning tool allowing users to ‘train’ a model which recognises structures more robustly than the traditional intensity of variance thresholding.
Imaris (Oxford Instruments)
Imaris is a leading interactive microscopy image analysis software package with excellent visualisation and rendering capability for 3D datasets such as those acquired on the Dragonfly Confocal or Nikon A1+ confocal. We are licensed for the Imaris Essentials Package which includes:
- 3D Surface Rendering and Generation of Snapshots and Animations
- Detection of Objects and Measurements of Size, Counts, Intensity etc.
- Distance Measurements, Spatial Interactions and Colocalization
- Machine Learning Object Classification
- Plotting Tools and Data Mining
Read more: Imaris Essentials
NIS Elements (Nikon)
Nikon’s analysis software for 3D rendering, image processing and feature quantitation of images captured on the Nikon A1+ confocal. Includes the Deconvolution module.
Volocity (PerkinElmer)
3D image visualisation and analysis software specifically for images generated on the Ultraview Confocal Microscope. Render objects in 3D, measure fluorescence intensities and colocalisation, and export data for further analysis.
Free software – also available for download to Personal PCs
FIJI/ImageJ
ImageJ is public domain software for processing and analysing scientific images. FIJI includes many useful plugins contributed by the global image analysis community.
Imaris Viewer
Free visualisation software to view, adjust display settings and export images acquired on the Dragonfly and Nikon Confocal microscopes.
NIS Elements Viewer
Free version of NIS elements for viewing, adjusting and saving images acquired on the Nikon A1+ Confocal Microscope.
The Histology suite contains the following equipment for processing paraffin fixing or frozen biological tissue, with all basic reagents to operate the equipment supplied by our Facility.
Leica paraffin processing and embedding station
The Leica TP1020 is a benchtop tissue processor designed to provide fixation, gentle dehydration, clearing and infiltration of various histological tissue samples with paraffin wax. Samples are loaded into embedding cassettes, then into a basket, and automatically moved through stations containing fixatives, alcohol, solvents, and paraffin wax.
The Leica EG1150H is a modern paraffin embedding station with a microprocessor control system designed to embed histological tissue specimens in molten paraffin. The instrument melts solid paraffin and maintains the required temperature for sample embedding. Paraffin is poured into embedding moulds where the specimens are placed, and the temperature of embedding cassettes, specimens, moulds and required forceps are maintained.
The Facility provides histology cassettes, paraffin wax, research-grade ethanol and xylene.
Leica Microtome
This instrument is a manually operated rotation microtome designed to create thin sections of tissue specimens embedded in paraffin for histological purposes. The instrument is set to a sectioning thickness of 1 – 60 μm, to produce a wax ribbon containing tissue samples which are adhered onto slides using the associated water bath and slide dryer.
Leica cryostats
We have 2x Leica CM1520 cryostats, which are manual microtomes that maintain cryogenic temperature (typically around −15 to −40 °C) of samples, thus enabling reliable and safe cutting of accurate frozen tissue sections 5 to 60um thick for mounting onto microscope slides.
H&E staining set
We have a ventilated station designed specifically for routine Haematoxylin and Eosin staining. The staining set contains 12 troughs with lids, 6x 25 slide racks and slide drying racks. We supply and renew all solvents and solutions, including Ethanol, Xylene, Gills No.2 Haematoxylin, Alcoholic Eosin, Scott’s Bluing solution, Acid Ethanol and Entellan mounting media.
360° Microscopy facility
Immerse yourself in a 360° virtual experience of our state-of-the-art Microscopy and Imaging Laboratory facility.
Further information
Training is a pre-requisite to independent access to the Facility, and all prospective users must complete training regardless of experience on similar instruments.
Microscopy and Histology Facility
To use our Microscopy and Histology Facility, you will need to follow these steps:
- Complete our Facility inductions (to organise your induction email CurtinMRI@curtin.edu.au)
- Email michael.nesbit@curtin.edu.au, Senior Technical Officer (STO) and provide a brief outline of your project, how you plan to use microscopy in your research, and anticipated timelines, and attach relevant references.
- Attend the first-user meeting. Depending on user experience and the nature of the experiment, more discussions may be required before commencing training. The STO will provide some learning resources to ensure a theoretical understanding of the instruments ahead of use.
- Microscopy Facility Induction
- The STO will introduce the user to Facility rules, safe work procedures and operation of the Olympus Widefield Fluorescent Microscopes to capture images of training slides. Training is in small groups or 1:1. Allow about 45-60 minutes.
- Access will only be granted after prospective users return a signed training acknowledgement.
Histology Suite Training
The STO will introduce the user to facility rules, safe work procedures and basic operation of cryostats, microtomes and tissue processing equipment and some basic troubleshooting. Training is in small groups or 1:1. Allow about 20-30 minutes.
Full cryostat or microtome training should be conducted by the user’s supervisor. Booking access will not be approved until the supervisor signs off the new user to operate the equipment safely.
Confocal Microscopes
Each instrument has a separate training program. Training is 1:1 and involves hands-on training with practice fluorescent slides to introduce:
- Start-up and shutdown procedures
- How to acquire good quality data to address the research question
- Imaging parameters using the user’s sample, including appropriate controls
- Basic troubleshooting and how to look after the microscope to ensure longevity.
- The first training session usually takes about 3 hours. Subsequent sessions should be organised with the STO until the user is deemed competent to operate the equipment independently.
Zeiss Axioscan Z1 Digital Slide Scanner
Training takes 1 to 3 hours, depending on the complexity of imaging requirements and will be performed with the users’ samples.
Contact the STO to discuss sample preparation, staining and imaging requirements well before training.
Note: This equipment is designed for large experiments. Staining protocols should be optimised/validated on other platforms before using this microscope.
Access to platform-specific online booking calendars is reserved for trained users only. If you have not received access to the microscopes, histology suite and/or the analysis computers after attending training, please contact michael.nesbit@curtin.edu.au.
All shared equipment-generated data should acknowledge our Microscopy and Histology facility. e.g. Confocal microscopy analysis was performed using the Curtin Medical Research Institute Microscopy and Histology Shared Resources Laboratory with the assistance of Mr Michael Nesbit.
In addition, where any member of our Microscopy and Histology facility has made substantial intellectual contributions to the design of your experiment, they should qualify among the authorship list.
Open hours
Appointments for training with our Microscopy and Histology facility staff are available 9am-6pm Monday to Friday (excluding Curtin-observed public holidays).
Trained users can access the analysis platforms 24/7.
Contact and feedback
If you have an enquiry or want to provide feedback, please contact:
Dr Rob Steuart
CurtinMRI Facilities Manager
+61 8 9266 2342
R.Steuart@curtin.edu.au
Michael Nesbit
Senior Technical Officer
+61 8 9266 2201
Michael.Nesbit@curtin.edu.au
